In this article, we will explain what the “HCOOCH CH₂ H₂O” reaction means, how it works, and why it matters. We use simple words and clear steps so any reader can follow.
1. What is “HCOOCH CH₂ H₂O”?
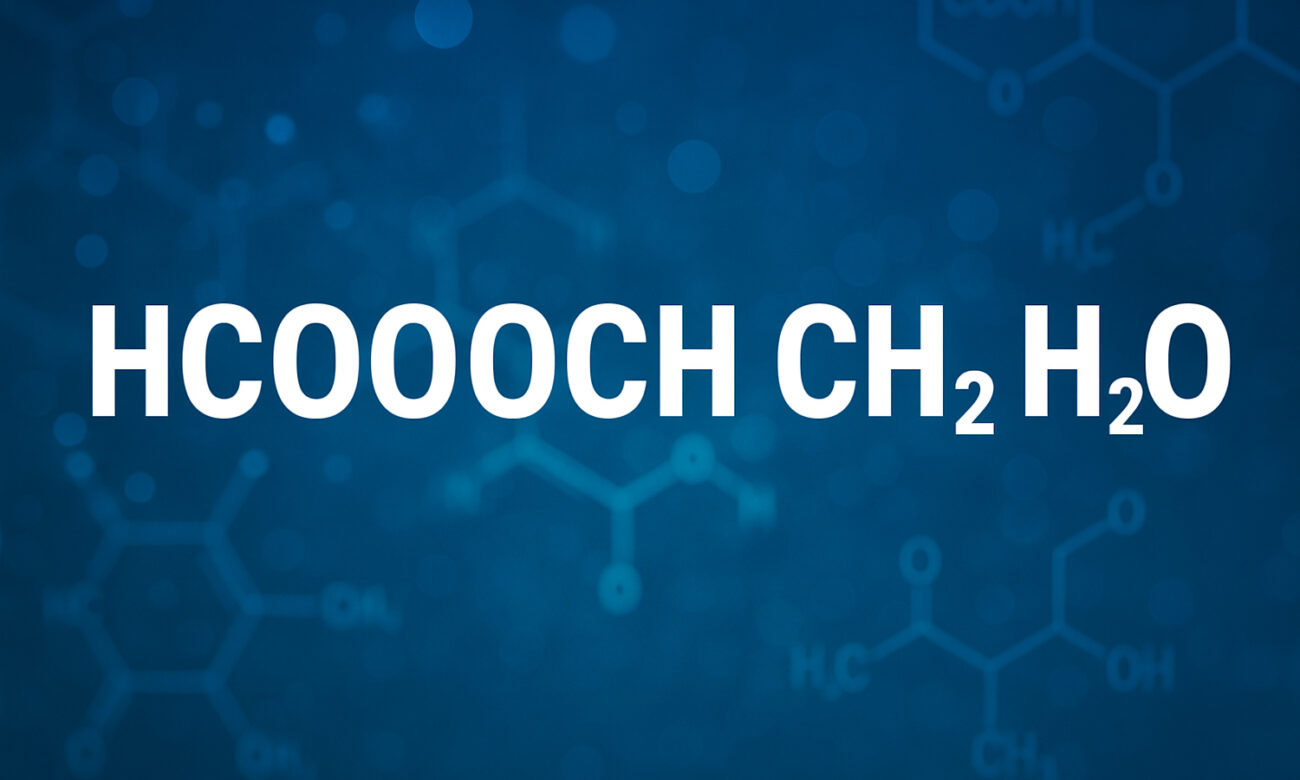
The phrase HCOOCH CH₂ H₂O is not a standard chemical formula. But many chemists interpret it as a shorthand or a miswritten form of the reaction between methyl formate (HCOOCH₃) and water (H₂O). In clearer terms:
- Methyl formate has formula HCOOCH₃.
- The reaction with water (hydrolysis) gives formic acid (HCOOH) and methanol (CH₃OH).
So the reaction is often written:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This is an ester hydrolysis reaction (an ester is a kind of organic chemical). It is a common reaction in chemistry.
2. Why is this reaction important?

There are several reasons why the hydrolysis of methyl formate is important:
- Production of formic acid
Many industrial processes use this reaction to make formic acid. - Recycling methanol
The methanol that appears as product can be reused in the process. This helps reduce waste and cost. - Chemical equilibrium and control
The reaction is reversible. Under some conditions, formic acid and methanol can react back to methyl formate (esterification). So industrial setups must control conditions to push the reaction in the forward direction. - Green chemistry and applications
Formic acid has uses in agriculture, leather tanning, cleaning, and as a building block in other chemicals. Also, methyl formate has roles in research on hydrogen storage or energy carriers.
3. How the reaction works (mechanism in simple terms)
Let me explain step by step, in easy language:
- Activation / Catalyst
Often, an acid or some catalyst helps the reaction start. This makes the carbonyl part (C=O) more open to attack. - Attack by water
A water molecule attacks the carbon atom in the ester (the carbon in the “HCOO” part). This forms a temporary “tetrahedral intermediate,” which is just an “in-between” structure. - Break the bond
The bond between the ester’s oxygen and the carbon breaks. That releases methanol (CH₃OH). The remaining part becomes formic acid (HCOOH). - Equilibrium and reverse reaction
Because the reverse (esterification) is possible, conditions are chosen to favor the forward reaction (toward acid + alcohol).
In industry, they often do things to shift the balance, like removing methanol as it forms. That drives the reaction forward.
Also, kinetic studies (which measure how fast steps happen) show that with some additives, the reaction has two phases, and water amount, stirring, temperature all matter.
4. Industrial process (how engineers do it at large scale)
Here is how the reaction is used in big chemical plants:
- First, companies often make methyl formate by reacting methanol + carbon monoxide (CO) in presence of a base (e.g. sodium methoxide).
- Then, methyl formate is sent to a hydrolysis reactor, where water is added. The reaction is done at elevated temperature (around 90–140 °C) and moderate pressure.
- In many designs, they introduce vapor streams of methyl formate to help strip away methanol from the mixture. This helps push the reaction forward (less reverse reaction).
- After reaction, they separate the mixture by distillation. Unreacted methyl formate or methanol can be recycled.
- They aim to get formic acid at reasonable concentration (for example 85 %) for sale.
Some designs do hydrolysis at 90–140 °C and 2–7 bar pressure, while passing methyl formate vapor to remove methanol, in a countercurrent setup.
Other designs note that using a molar excess of methyl formate and flashing (rapid vaporization) helps avoid re-esterification.
So the industrial method is a careful balance of temperature, pressure, feed ratios, and separation steps.
5. Factors that affect the reaction
Here are key factors engineers and chemists watch:
- Temperature: Higher temperature speeds reaction but may favor reverse reaction (esterification).
- Concentrations: More water (excess) helps push toward acid + alcohol.
- Removal of product: If methanol is removed quickly, the reaction favors forward path.
- Catalyst or acid: Presence of acid (or formic acid itself) helps accelerate the reaction.
- Stirring / mixing: Good mixing avoids local concentration differences.
- Equilibrium constant: The reaction equilibrium constant is not always strongly in favor of the products; so you need to use strategies to shift the balance.
A kinetic study showed that in absence or presence of complexing agents, reaction rates differ and water amount is important.
6. Benefits and challenges
Benefits
- Good yield of formic acid when process is well controlled.
- Methanol can be recycled, reducing waste.
- Widely used and well studied.
- It ties into green chemistry ideas: formic acid is a useful chemical for many industries.
Challenges
- Reverse reaction (re-esterification) is always a risk.
- Separating methanol, methyl formate, water, formic acid mixtures is tricky.
- The equilibrium is not extremely favorable; so you need steps to push the reaction forward (like removing methanol or using excess reagents).
- High temperature or pressure adds cost and complexity.
- If conditions are not well controlled, losses or side reactions may occur.
7. Real-world uses and future directions
- Formic acid uses: leather tanning, agriculture, cleaning agents, chemical building blocks.
- Methanol reuse: The methanol from the reaction is reused in the same plant, making the process cyclic.
- Hydrogen storage / energy: Some recent research explores using methyl formate and formic acid as carriers in hydrogen energy systems.
- Green improvements: Scientists are working on catalysts, milder conditions, better separation to make the process greener and cheaper.
8. Summary

To sum up, the phrase “HCOOCH CH₂ H₂O” is most likely a miswritten or shorthand of the reaction:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
That is the hydrolysis of methyl formate into formic acid and methanol. This reaction is valuable in industry for producing formic acid. But it also comes with challenges—control of equilibrium, separation of products, and managing reverse reactions.
When done right, it is efficient and useful, especially when methanol is recycled. Advances in catalysts and process design continue to improve its performance and sustainability.
Conclusion
The reaction known as HCOOCH CH₂ H₂O is best understood as the hydrolysis of methyl formate with water. It is a simple but important chemical process that makes formic acid and methanol. This reaction is used in many industries because it gives useful products and can be done safely and efficiently with the right conditions.
By controlling temperature, pressure, and water amount, scientists can make the reaction faster and avoid unwanted side reactions. Reusing methanol also helps reduce waste and supports green chemistry goals.
Frequently Asked Questions (FAQs)
- What is methyl formate?
Methyl formate is the methyl ester of formic acid. Its formula is HCOOCH₃. It is a colorless liquid and used in organic chemistry. - What does hydrolysis mean?
Hydrolysis means breaking a chemical bond by reaction with water. In this case, the ester bond in methyl formate is broken by water. - What do you get from the reaction?
You get formic acid (HCOOH) and methanol (CH₃OH). - Is the reaction reversible?
Yes, under certain conditions formic acid and methanol can react back to methyl formate (called esterification). - What conditions help the reaction go forward?
Using extra water, raising temperature (within limits), and removing methanol as it forms all help push toward acid + alcohol. - What temperature and pressure are used industrially?
Common industrial conditions are about 90–140 °C and pressures between 2 and 7 bar in many designs. - Why remove methanol during reaction?
Removing methanol shifts the equilibrium and helps the reaction go more toward formic acid. - What is a challenge of this reaction?
The biggest challenge is preventing the reverse reaction and doing good separation of the mixture of chemicals. - Can this reaction be green or eco-friendly?
Yes. By improving catalysts, reducing energy use, and recycling methanol, it can be made more sustainable. - What are future uses or research directions?
Using methyl formate / formic acid in hydrogen storage, improving catalysts, and better process designs are key future areas.
Click for more amazing info. News Cora